Table of Contents
ToggleIn the early 20th century, a curious scientist named Ernest Rutherford turned the world of atomic physics upside down with a simple yet groundbreaking experiment. Picture him in a lab, firing alpha particles like a kid with a slingshot, only to discover that atoms aren’t just tiny blobs of goo. Instead, they have a hidden structure that would make a Swiss cheese jealous!
Overview of Rutherford’s Experiment
Ernest Rutherford conducted a pivotal experiment in 1909 known as the gold foil experiment. Researchers directed alpha particles at a very thin sheet of gold foil. Most of these particles passed through the foil with minimal deflection, suggesting a predominantly empty space within the atom. A small fraction of the particles, however, experienced significant deflections. This surprising outcome indicated that a dense nucleus existed at the center of the atom.
The results of this experiment transformed the atomic model. Previously, scientists viewed atoms as solid, indivisible spheres. Rutherford’s findings contradicted this notion, revealing that atoms contain a nucleus surrounded by electrons. The nucleus hosts protons and neutrons, leading to a more complex understanding of atomic structure. Scientists recognized this nucleus as the core where most of the atom’s mass resides.
This experiment laid the foundation for future research in nuclear physics. Researchers built on Rutherford’s work, leading to the development of the modern atomic model. This model further elucidates electron arrangements and energy levels around the nucleus. Rutherford’s experiment highlighted the necessity of examining atomic particles closely, leading to advances in chemical reactions and radioactivity studies.
Rutherford’s contributions significantly influenced scientific thought at the time. His experiment inspired scientists worldwide to rethink atomic theory. New frameworks emerged, prompting the exploration of subatomic particles and their interactions. Ultimately, this understanding opened avenues for advancements in various scientific fields, including chemistry, physics, and materials science.
Key Discoveries
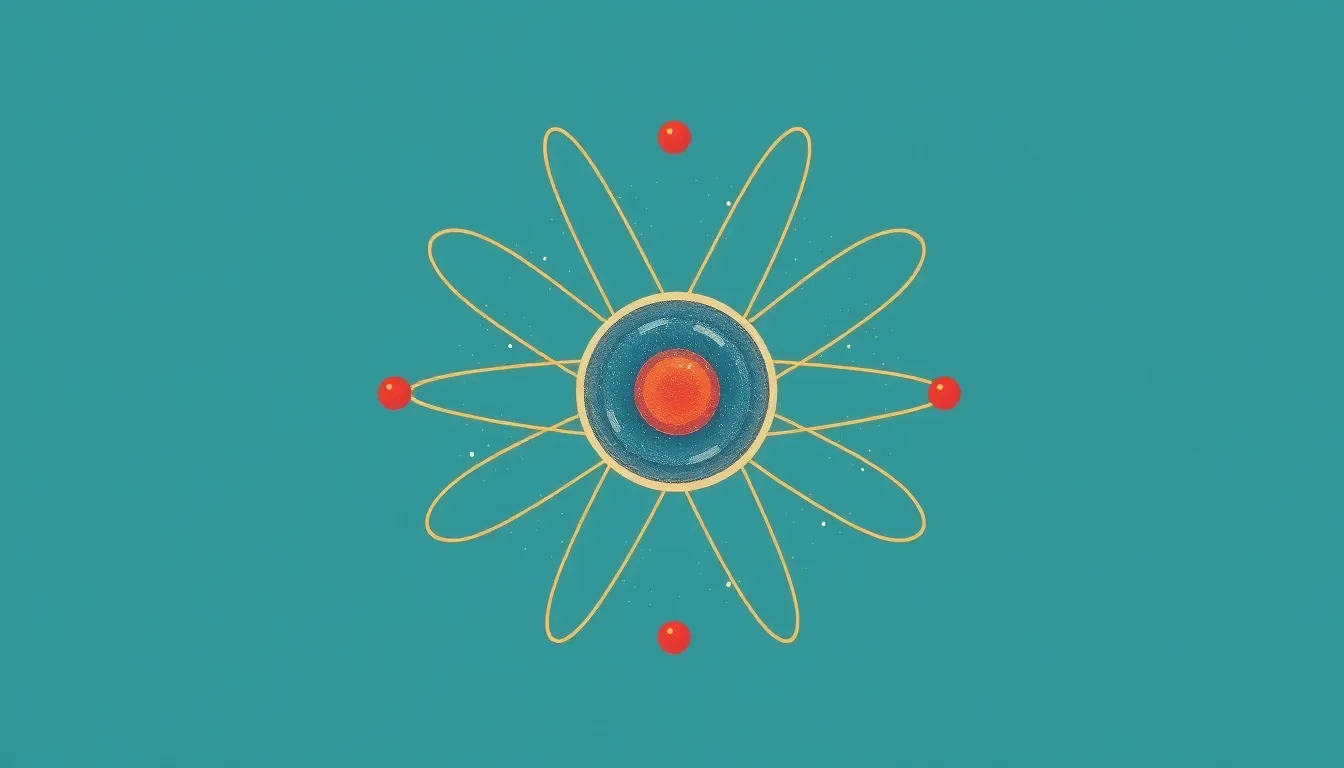

Rutherford’s experiments led to several groundbreaking discoveries that reshaped atomic theory. His insights primarily focused on the nucleus and atomic structure.
The Nucleus
The nucleus sits at the center of an atom. It contains protons and neutrons, which contribute to the atom’s mass. Most alpha particles passed through the gold foil, demonstrating that atoms consist of mostly empty space. A small fraction of particles deflected significantly, implying a dense, positively charged core. This finding established the nucleus as a key component in understanding atomic behavior.
Atomic Structure
Rutherford’s work revealed a complex atomic structure. Electrons orbit the nucleus, creating a model that differs from the previous solid sphere conception. The nucleus accounts for nearly all atomic mass, while electron clouds occupy the majority of atomic volume. This arrangement prompted scientists to investigate how electrons interact and influence chemical properties. Understanding atomic structure paved the way for advancements in chemistry and physics, guiding subsequent research in these fields.
Implications of Rutherford’s Findings
Rutherford’s discoveries significantly altered the landscape of atomic science. His insights illuminated the complexities of atomic structure, revealing the nucleus at the core of every atom.
Influence on Modern Physics
Modern physics owes much to Rutherford’s work. His experiments prompted the reevaluation of atomic models. Discovering the nucleus led to further exploration of atomic interactions. Many physicists built upon his findings, developing theories on nuclear forces and particle physics. Innovations in technology, such as nuclear energy and medical imaging, also derive from Rutherford’s foundational contributions.
Impact on Atomic Theory
Rutherford’s findings reshaped atomic theory fundamentally. By introducing the nuclear model, he challenged existing notions of solid atoms. This new perspective led to a deeper understanding of electron behavior and chemical bonding. Subsequent theories, such as quantum mechanics, emerged due to his insights. The shift from a simplistic view of atoms to a complex structure has driven various research fields forward.
Rutherford’s discoveries revolutionized the understanding of atomic structure. His gold foil experiment unveiled the nucleus as the core of the atom, fundamentally changing the landscape of atomic physics. This shift not only contradicted previous models but also paved the way for future research in nuclear physics and chemistry.
The implications of his work extend far beyond the laboratory. Innovations in technology and advancements in various scientific fields owe much to Rutherford’s insights. By challenging the notion of solid atoms, he laid the groundwork for modern atomic theory, influencing how scientists approach the study of matter and energy. Rutherford’s legacy continues to inspire exploration and discovery in the realms of science.







